The small molecule acetylome
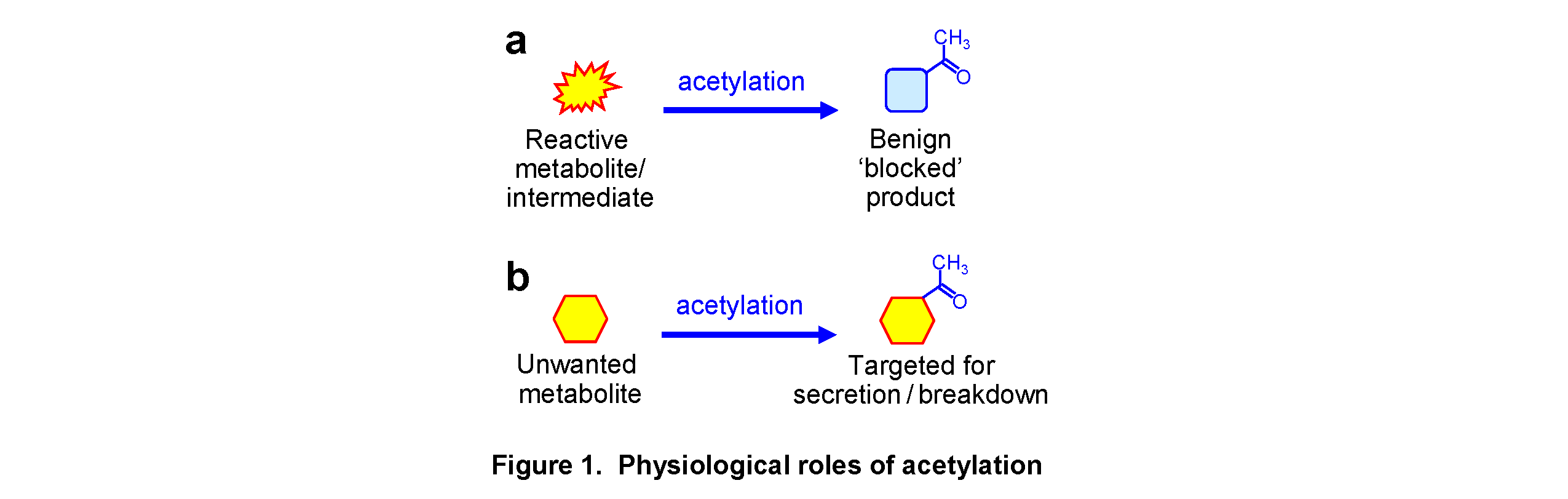
Acetyltransferases catalyze the transfer of an acetyl group from acetyl-CoA to an acceptor such as a small molecule. Most characterized small molecule acetyltransferases have metabolic support roles. A reactive or toxic intermediate can be acetylated to make it less reactive (Figure 1a). A good example is in arginine synthesis – if the glutamate semialdehyde intermediate wasn’t blocked with an acetyl group it would spontaneously cyclize to the proline synthesis intermediate, P5C. This is analogous to an organic chemist blocking and deblocking a reactive group during chemical synthesis. An unwanted metabolite can be acetylated to target it for secretion or breakdown (Figure 1b). A nice example of this is seen in the classical Lac operon. LacY can pump lactate into cells to toxic levels. LacA is a low affinity acetyltransferase that acetylates excess lactate, which is then selectively excreted. LacA thus acts like a “pressure relief valve” preventing lactose toxicity. This appears to be a common strategy in bacteria – a similar low affinity acetyltransferase exists to prevent maltose toxicity.
Most acetyltransferases are uncharacterized – a typical microbe has dozens and a typical plant has ~200 uncharacterized small molecule acetyltransferases that probably have metabolic support roles. Bioinformatics doesn’t provide many clues as to which substrate they act on. A technique that could identify all acetylated compounds (i.e. the ‘small molecule acetylome’) in an organism would reveal unwanted/toxic compounds that acetyltransferases have evolved to deal with. In essence, flagging acetylated compounds flags damaged/reactive compounds. These compounds are de facto targets for metabolic support systems that can be used in a variety of applications.
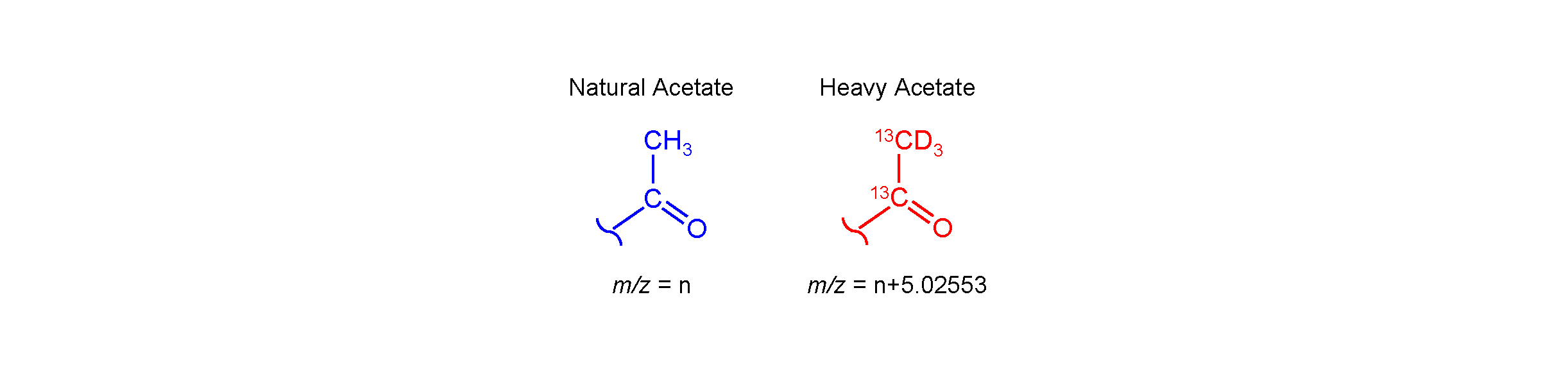
Acetyl-CoA, the cosubstrate of all acetyltransferases, can be formed by directly transferring acetate to CoA. In principal, labeled acetate supplied to plants or microbes could be incorporated into acetyl-CoA and transferred to acetylated compounds. Fully labeled heavy acetate (13C2D3) is +5.02553 amu larger than natural acetate; a heavy-acetylated compound would likewise be +5.02553 amu larger (see below). By comparing LCMS metabolic profiles of organisms supplied with heavy acetate to those given natural acetate, peaks can be identified that have a distinct +5.02553 mass shift in the heavy acetate-grown samples – these are almost certainly acetylated compounds. Using this strategy, acetylated compounds can be easily flagged. Without isotope-enhanced metabolomics, identifying acetylated compounds is not feasible because there are no defining MS features to diagnose an acetyl group.
TCA cycle intermediate damage and repair
Cellular thiols such as cysteine spontaneously and readily react with the respiratory intermediate fumarate, resulting in the formation of stable S-(2-succino)-adducts. Fumarate-mediated succination of thiols increases in certain tumors and in response to glucotoxicity associated with diabetes. Therefore, S-(2-succino)-adducts such as S-(2-succino)cysteine (2SC) are considered oncometabolites and biomarkers for human disease. Recently we discovered a disposal route for 2SC in Bacillus subtilis (see reference here). The first step is N-acetylation of 2SC followed by an oxygenation that we propose results in the release of oxaloacetate and N-acetylcysteine, which is deacetylated to give cysteine.
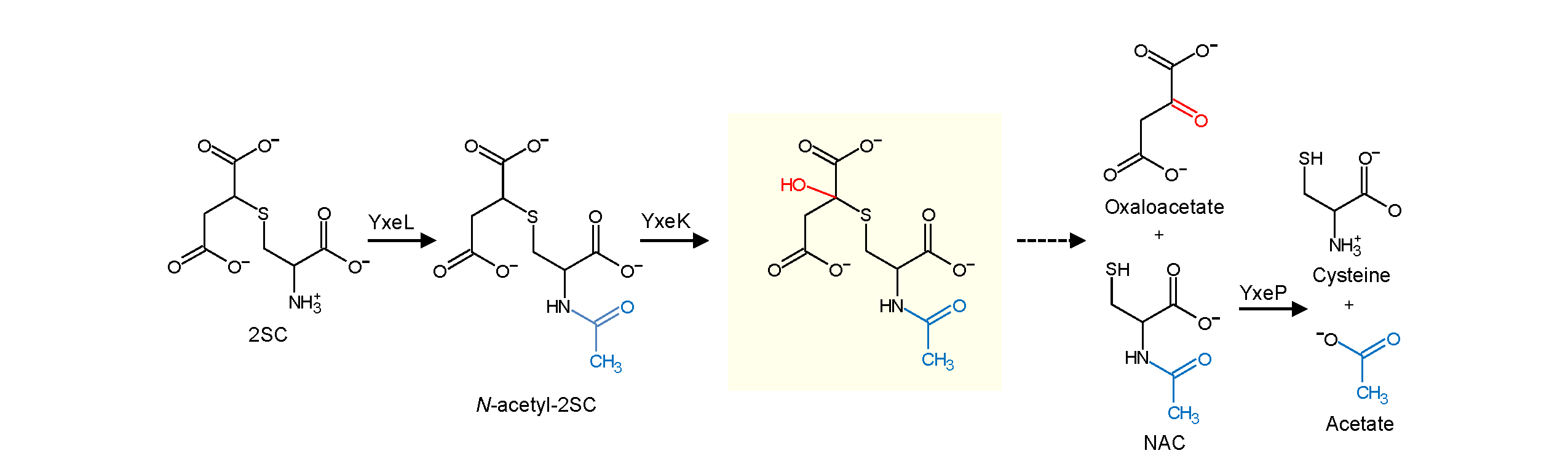
Using comparative genomics, we have identified two possible alternative pathways to 2SC degradation in microbes and plants. We are currently planning to characterize these two alternative pathways and test whether 2SC degradation pathways can be used in medical or synthetic biology applications.